
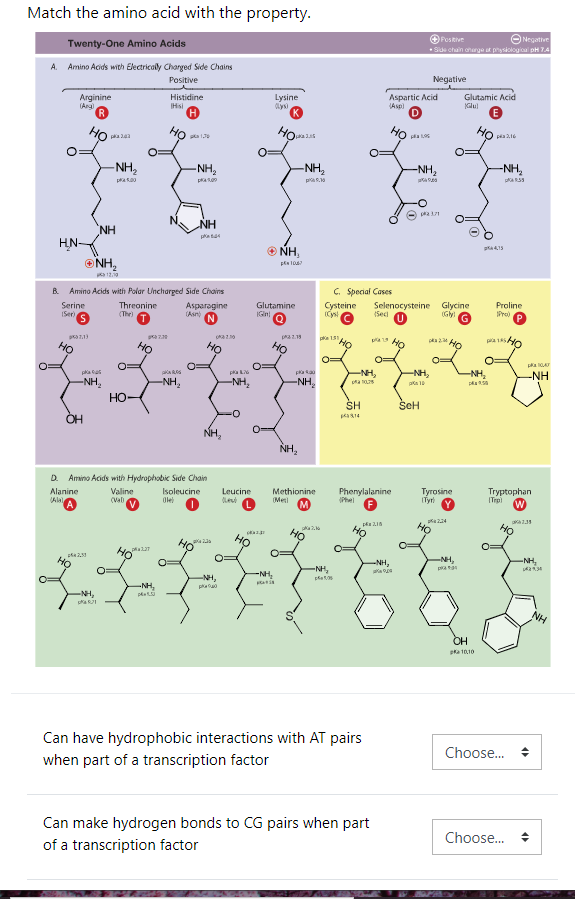
The pH of pure water is 7, precisely in the middle. Hydrogen molecules are why bases and acids are often measured in pH levels (pH stands for "potential of hydrogen") as related to pure water. A strong base molecule can deprotonate, or take the proton, of a weaker acid such as water. Acids are the compounds that donate a hydrogen ion (H+) to a base, while a base compound is one that can remove a proton (H+ is a proton) from an acid. A base molecule or compound is the opposite of an acid. In chemistry, we're talking about molecules (which are themselves made up of atoms). base when it comes to a chemistry standpoint. With that structure explained, let us next generally cover acid vs. what color your lava lamp is), but these side chains also determine whether or not an amino acid is basic or acidic. We illustrate it this way because it's that glass lava vessel that not only determines what kind of amino acid you have (i.e. And the little decorative cap on top? Your single hydrogen atom. The light bulb in the center? That is your central alpha carbon. With 20 different color combinations, you have enough to represent all 20 amino acids. Maybe one vessel has blue water and green lava, while another one has purple water with yellow lava, while yet another has blue water combined with yellow lava. With a long row of 20 identical lava lamps, imagine that the only thing that would make any of them unique is the color combination inside its radical glass lava vessel. Each one is made up of a light bulb in the center, a power cord, a stand, and a cap. Picture it, if you will, as a group of lava lamps. The R group you can think of as the Radical group, as it is different and unique on each and every amino acid, of which there are 20 main ones in the human body. This is the same structure of all amino acids, until the last piece of the puzzle joins the rest: the R group side chain. Each amino acid is made of a central alpha carbon atom (Cα), and attached to that central atom are three molecular structures, also known as functional groups: one is a carboxyl group (-COOH), the second is an amino group (-NH2), and the third is a single hydrogen atom (H). To understand what differentiates amino acids, you'll have to understand their structure and what they have in common.
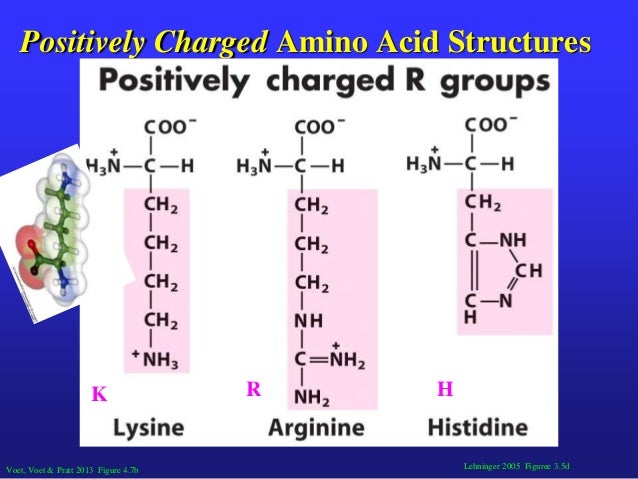
Now that you know the amino acids we'll be talking about, let's move on to an illustrative example of what an amino acid's structure looks like. Your essential amino acids are the ones you need but cannot produce yourself, and so must be gained either from your diet or via supplementation. There are 11 of these amino acids, and they include the following: Your nonessential amino acids are the amino acids your body can create on its own as a byproduct of normal functioning. In short, when it comes to protein synthesis and literally building new muscle, you cannot do it without your amino acids. NonessentialĪmino acids are the buildings blocks of protein in your body.

We'll provide the definitions and explanations you seek, including what are amino acids, what makes some of them acidic and some of them basic, and why that matters when it comes to the chemistry of the human body. This article will quickly inform you of the difference between acidic and basic amino acids.


 0 kommentar(er)
0 kommentar(er)
